If you are in the manufacturing business, be it food and beverages, pharmaceuticals, cosmetics or chemicals, it is compulsive to follow statutory cGMP compliance. Failing to meet the statutory guidelines result in violations.
But, how should one meet this? Here comes the purpose of our blog. Before delving into the answer of the question, let’s first understand what cGMP is and what its requirements are?
So, what is cGMP?
cGMP stands for Good Manufacturing Practices, which contains a set of regulations enforced by the US FDA. The ‘C’ signifies Current, reminding manufactures to employ technologies, innovative approaches and systems that are up-to-date for the production processes.
cGMP lists the mandates for following of the right practices of manufacturing. And, the companies who fail to follow these stated mandates get warning letters from the FDA and their products are called off from the market. This in turn badly impacts customer loyalty and taints the brand image too. Hence, it’s in the best interest of any organization to follow Good Manufacturing Practices in order to keep trouble by the FDA, customer and competitors at bay.
But, why is it needed?
Generally, quality control testing methods are employed to ensure the quality of goods produced, but the testing alone isn’t sufficient. Reason being, the tests are performed on a small sample size out of tons of the materials. So, it is not necessary that if a small batch sample qualifies the QC test, the rest of the goods also do. Therefore, it is obligatory according to the FDA that all the manufacturing and packaging processes should occur as per the practices and conditions illustrated in cGMP guidelines. Industries employ cGMP compliance to make quality the synonym of their product and inculcate the practice of developing best quality products at every step.
Adhering to cGMP compliance guarantees a lot many things. Major being- adequate tracking of the manufacturing process along with their regular monitoring and control.
What does it consist of?
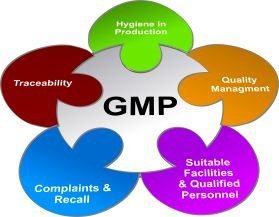
cGMP compliance includes the basic necessities like maintaining hygienic conditions for production, strict watch on the formulas and quantity of sensitive ingredients, using machines and equipment that are sterilized and calibrated, hiring qualified and trained labors, following reliable and safe processes, etc. In case the manufactured goods don’t meet the quality standards, then traceability and recalls come under the scope of cGMP.
That mentioned, we come back to our question – ‘How one should meet the cGMP?’ The answer is- Implement an ERP. It is the best way to ensure that good manufacturing practices are followed across the length & the breadth of your organization.
ERP – the best way to adhere to cGMP
Considering the significance of the cGMP compliance in today’s time, it is essential that enforcement of the regulations like producing standard quality products in prescribed ambience should happen smoothly, coherently while also fitting into the budget. It not only helps you gain confidence of the Food and Drug Administration (FDA), but also the trust of your customers as a result of the quality goods delivered.
An ERP can be an efficient tool to reduce the chances of cGMP requirement violation and compromise on quality. However, not any ERP out there in the market can serve the purpose. The need is of an ERP that by-design follows cGMP guidelines and ensures inculcation of same in your processes too. An ERP that ensures the following requirements can prove to be a perfect fit.
Ensuring Quality
The first aspect that an ERP targets is ensuring quality standards, by allowing setting up user defined QC tests, and selecting processes on which they should be applied. No process can be accomplished unless QC has been performed on it. Marking of selected and rejected batches is done automatically based on the results, after the QC tests are performed. Movement of material along the supply chain is governed based on the quality statuses. Batches & products are effectively inspected, and the non-conformance and CAPA records are generated in case of any deviation. SOPs can be associated with baseline formulas, production batches as well as packaging materials to provide step-by-step instructions reducing administrative tasks, speeding approvals and simplifying processes.
Ensuring Security
Another important feature, an ERP offers is- security. In order to follow cGMP compliance, it is crucial to lock the composition of your formula so that your formulas are secured and uniform manufacturing is practiced always. ERP ensures security which not only takes care of the formulas, but also of packaging specifications and QC results. Any insertion, deletion or modification in system specific records require approval from the authorized personnel(s) before getting implemented. All the records are time and user stamped with access to only the designated users.
Ensuring Compliance
ERP generates and captures lot numbers right from the time of receiving of ingredients and packaging materials to customer shipments. It ensures complete tracking of the product across the entire supply chain. This way, an ERP helps you to quickly perform the recalls and trial recalls to identify the suspect products, cutting down the chances of contaminated goods getting out of your premises. Moreover, the software’s ability to produce recall letters to customers and suppliers is an added bonus.
In this situation of “comply-or die”, having traditional tools like outdated, generic or a discrete ERP can directly impact not only the growth but the survival of the organization. An ERP, rather an ERP with capabilities to ensure cGMP, that too without any workarounds, can keep away cGMP violation letters. Instead, an ERP, through its various capabilities, can help build better customer relationship, shape customer loyalty and result in repeat business- important for your profitability.
With so many violation letters being sent out every year, it’s a wakeup call for manufacturing businesses to switch to an ERP software asap, and leverage its numerous benefits along with meeting cGMP.