Data integrity is the cornerstone of compliance in the pharmaceutical industry. In a rapidly growing and challenging market, where supply chain disruptions and tightening regulatory laws put stress on the industry, data integrity is not only a compliance mandate, but also crucial for maintaining consumer trust.
This blog explores the critical role of data integrity in pharmaceutical compliance and why organizations must prioritize it to remain competitive and compliant.
What is Data Integrity and Why Is It Important?
Data integrity ensures that an organization’s data is complete, consistent, accurate, and validated. It not only signifies the completeness and consistency of data, but also ensures its accuracy and serves as proof of data validation. Since consistent data is the result of a careful and vigilant environment that prevents data breaches, data integrity also encompasses data security.
The importance of data integrity increases as the volume of data grows. Major organizational decisions, such as predicting consumer behaviour and assessing market activity, are based on data. Therefore, ensuring data integrity is of utmost importance. In the pharmaceutical industry, where data directly affects millions of lives, no organization can afford to compromise on data integrity.
Before moving forward, let’s look at some of the key regulatory frameworks governing data integrity.
Regulatory Frameworks Governing Data Integrity
FDA’s 21 CFR Part 11 – U.S. Compliance for Electronic Records & Signatures
This regulation ensures that electronic records hold the same level of credibility as paper-based records when used for compliance with good practices (GxP).
Key Requirements:
- Validation of Systems: Electronic systems must be validated to ensure reliability and consistency.
- Audit Trails: Any modifications to records should be time-stamped and automatically logged.
- Data Access Controls: Role-based permissions must be established to prevent unauthorized access.
- Electronic Signatures: Digital approvals must be legally binding and associated with verified identities.
EMA Guidelines on Data Integrity – European Perspective
The European Medicines Agency (EMA) provides guidelines applicable to electronic and paper-based data management across manufacturing, testing, and distribution.
Key Principles:
ALCOA+ Compliance: EMA reinforces the ALCOA+ principles, ensuring data is:
- Attributable: Indicates who recorded the data.
- Legible: Easily readable and permanently recorded.
- Contemporaneous: Recorded in real-time as actions occur.
- Original: The first capture of data, not a copy or altered version.
- Accurate: Free from errors.
- Plus: Complete, Consistent, Enduring, and Available.
MHRA Data Integrity Guidance – UK Regulations
The UK’s Medicines and Healthcare Products Regulatory Agency (MHRA) released a comprehensive guideline on data integrity in March 2018. It focuses on regulatory expectations for both paper and electronic records across the product lifecycle.
Key Requirements:
- Governance Framework: Companies must define policies, training programs, and compliance monitoring mechanisms.
- Critical Thinking in Data Reviews: Manual data verification should involve contextual understanding rather than mere checklist compliance.
- Data Transfer Controls: If data is transferred between systems (e.g., from lab equipment to ERP), validation is required to ensure accuracy.
Why Is Data Integrity Important for Compliance?
As mentioned earlier, various regulatory bodies enforce strict guidelines to ensure data integrity across all pharmaceutical operations. Any deviation from these guidelines can lead to severe penalties.
Regulatory warnings and penalties can create a cascading effect, damaging brand reputation and eroding customer trust. Maintaining data integrity is fundamental to compliance, helping organizations confidently navigate the complexities of laws and mandates.
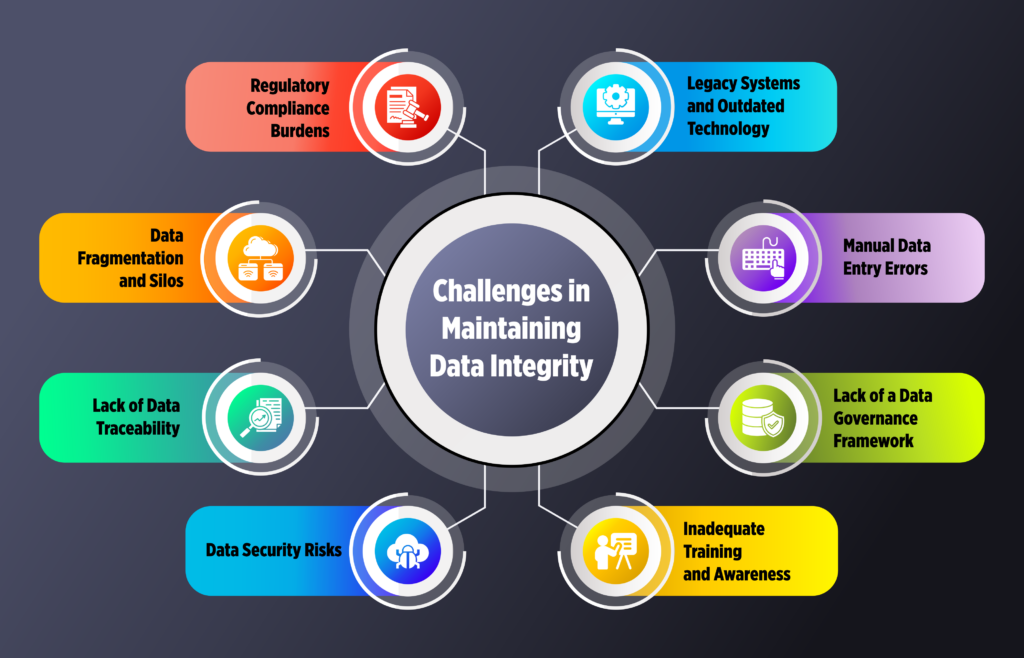
Challenges in Maintaining Data Integrity
Ensuring data integrity in the pharmaceutical industry is not without challenges. Some of the most common obstacles include:
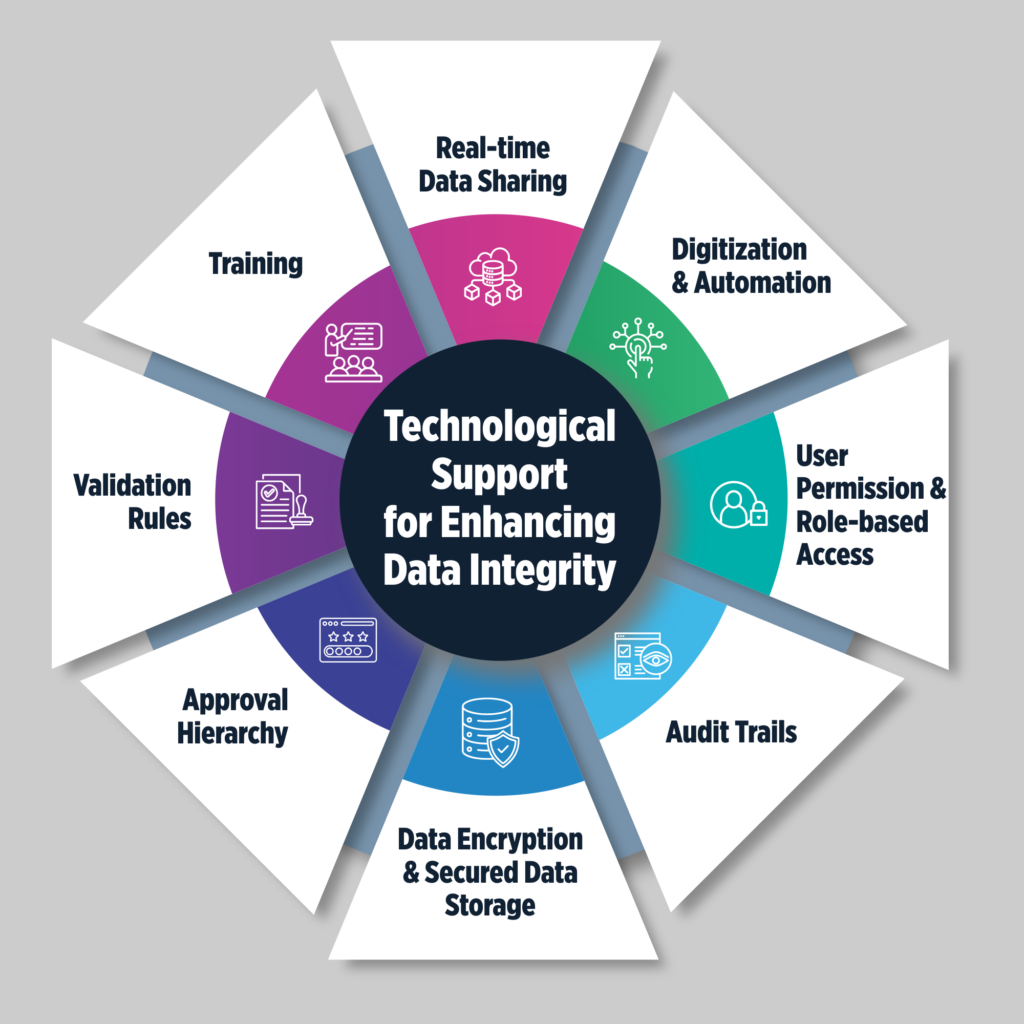
Technological Support for Enhancing Data Integrity
Finding solutions to these challenges is crucial for pharmaceutical organizations to thrive in an increasingly regulated market. Many organizations turn to technology, particularly pharmaceutical ERP software, to ensure data integrity. Let’s explore how these systems help maintain data integrity:
How ERP Software Supports Data Integrity?
- Real-time Data Sharing: ERP software centralizes data, eliminating silos and ensuring real-time updates across departments. This reduces ambiguity regarding data status.
- Digitization and Automation: By digitizing processes and automating redundant tasks, pharmaceutical ERP software minimizes manual intervention and reduces human errors.
- User Permissions & Role-Based Access: ERP systems enforce role-based access controls, ensuring that only authorized personnel can access sensitive data. Through Read, Write and None permissions, it governs who can only view while who can view and edit both.
- Audit Trails: Pharmaceutical ERP solutions maintain audit trails, tracking who made changes and when. This enhances transparency and accountability.
- Approval Hierarchy: ERP systems incorporate approval workflows, requiring higher-level validation before changes take effect. This adds an extra layer of security.
- Validation Rules: By implementing validation rules, ERP software ensures that only accurate and standardized data is entered into the system, reducing inconsistencies.
- Data Encryption & Secure Storage: Encryption protects data, both in transit and at rest, preventing cyberattacks. Secure storage safeguards the confidentiality and integrity of sensitive data.
- Training & Support: ERP software includes training programs to educate employees on best practices for data entry and management, ensuring clean data migration and maintenance.
Key Trends in Pharma Data Integrity
As pharmaceutical industries evolve, new technologies are emerging to strengthen data integrity. Some of the most promising advancements include:
Blockchain Technology
Blockchain provides a safeguard against data breaches and cyberattacks. Data recorded on a blockchain cannot be altered without the consent of all participating members. Additionally, decentralization ensures that data control is not limited to a single entity, promoting transparency and minimizing manipulation.
Artificial Intelligence & Machine Learning
With the increasing volume of data, managing accuracy becomes more challenging. AI and ML can help by detecting data integrity issues early. These technologies enhance data quality and safety while reducing human intervention and errors.
Conclusion
Data integrity is essential for maintaining compliance in the pharmaceutical industry. By ensuring information is accurate, consistent, and secure, organizations can meet regulatory requirements, safeguard patient trust, and uphold product quality—ultimately strengthening their market presence.
Although maintaining data integrity presents challenges, for which pharmaceutical ERP software offers a reliable solution. Among industry leaders, BatchMaster ERP for Pharma has stood out for over 40 years, serving global clients with robust compliance features.
Adhering to FDA, EMA, and other regulatory standards, BatchMaster ERP provides strong protection against cyber threats.
If you’re looking to enhance data integrity and compliance, we have the solution you need. To learn more, contact us by clicking here or email us at sales@batchmaster.co.in.